Influence of strong iron-binding ligands on cloud water oxidant capacity
Abstract
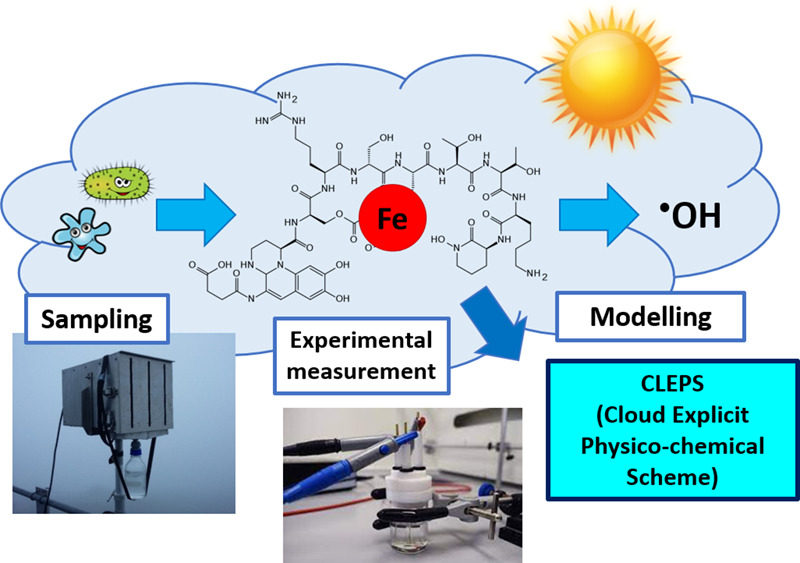
Iron (Fe) plays a dual role in atmospheric chemistry: it is involved in chemical and photochemical reactivity and serves as a micronutrient for microorganisms that have recently been shown to produce strong organic ligands. These ligands control the reactivity, mobility, solubility and speciation of Fe, which have a potential impact on Fe bioavailability and cloud water oxidant capacity.
In this work, the concentrations of Fe-binding ligands and the conditional stability constants were experimentally measured for the first time by Competitive Ligand Exchange-Adsorptive Cathodic Stripping Voltammetry (CLE-ACSV) technique in cloud water samples collected at puy de Dôme (France). The conditional stability constants, which indicate the strength of the Fe-ligand complexes, are higher than those considered until now in cloud chemistry (mainly Fe-oxalate). To understand the effect of Fe complexation on cloud water reactivity, we used the CLEPS cloud chemistry model. According to the model results, we found that Fe complexation impacts the hydroxyl radical formation rate: contrary to our expectations, Fe complexation by natural organic ligands led to an increase in hydroxyl radical production. These findings have important impacts on cloud chemistry and the global iron cycle.
Highlights
- 95% of iron is complexed by strong organic ligands, likely produced by microorganisms.
- Fe complexes stability constants are much higher than those used in cloud chemistry.
- The presence of strong organic ligands induces an increase in hydroxyl radical production.
- The analysis of sources and sinks of radical dotOH highlighted that complexed iron does not deplete HO2/O2−radical dot.
Reference
Aridane G. González, Angelica Bianco, Julia Boutorh, Marie Cheize, Gilles Mailhot, Anne-Marie Delort, Hélène Planquette, Nadine Chaumerliac, Laurent Deguillaume, Geraldine Sarthou. Influence of strong iron-binding ligands on cloud water oxidant capacity, Science of The Total Environment, Volume 829, 2022, 154642, ISSN 0048-9697, https://doi.org/10.1016/j.scitotenv.2022.154642.







