Identification of Polyunsaturated Fatty Acids Synthesis Pathways in the Toxic Dinophyte Alexandrium minutum Using 13C-Labelling
Abstract
The synthetic pathways responsible for the production of the polyunsaturated fatty acids 22:6n-3 and 20:5n-3 were studied in the Dinophyte Alexandrium minutum. The purpose of this work was to follow the progressive incorporation of an isotopic label (13CO2) into 11 fatty acids to better understand the fatty acid synthesis pathways in A. minutum. The Dinophyte growth was monitored for 54 h using high-frequency sampling. A. minutum presented a growth in two phases. A lag phase was observed during the first 30 h of development and had been associated with the probable temporary encystment of Dinophyte cells. An exponential growth phase was then observed after t30. A. minutum rapidly incorporated 13C into 22:6n-3, which ended up being the most 13C-enriched polyunsaturated fatty acid (PUFA) in this experiment, with a higher 13C atomic enrichment than 18:4n-3, 18:5n-3, 20:5n-3, and 22:5n-3. Overall, the 13C atomic enrichment (AE) was inversely proportional to number of carbons in n-3 PUFA. C18 PUFAs, 18:4n-3, and 18:5n-3, were indeed among the least 13C-enriched FAs during this experiment. They were assumed to be produced by the n-3 PUFA pathway. However, they could not be further elongated or desaturated to produce n-3 C20-C22 PUFA, because the AEs of the n-3 C18 PUFAs were lower than those of the n-3 C20-C22 PUFAs. Thus, the especially high atomic enrichment of 22:6n-3 (55.8% and 54.9% in neutral lipids (NLs) and polar lipids (PLs), respectively) led us to hypothesize that this major PUFA was synthesized by an O2-independent Polyketide Synthase (PKS) pathway. Another parallel PKS, independent of the one leading to 22:6n-3, was also supposed to produce 20:5n-3. The inverse order of the 13C atomic enrichment for n-3 PUFAs was also suspected to be related to the possible β-oxidation of long-chain n-3 PUFAs occurring during A. minutum encystment.
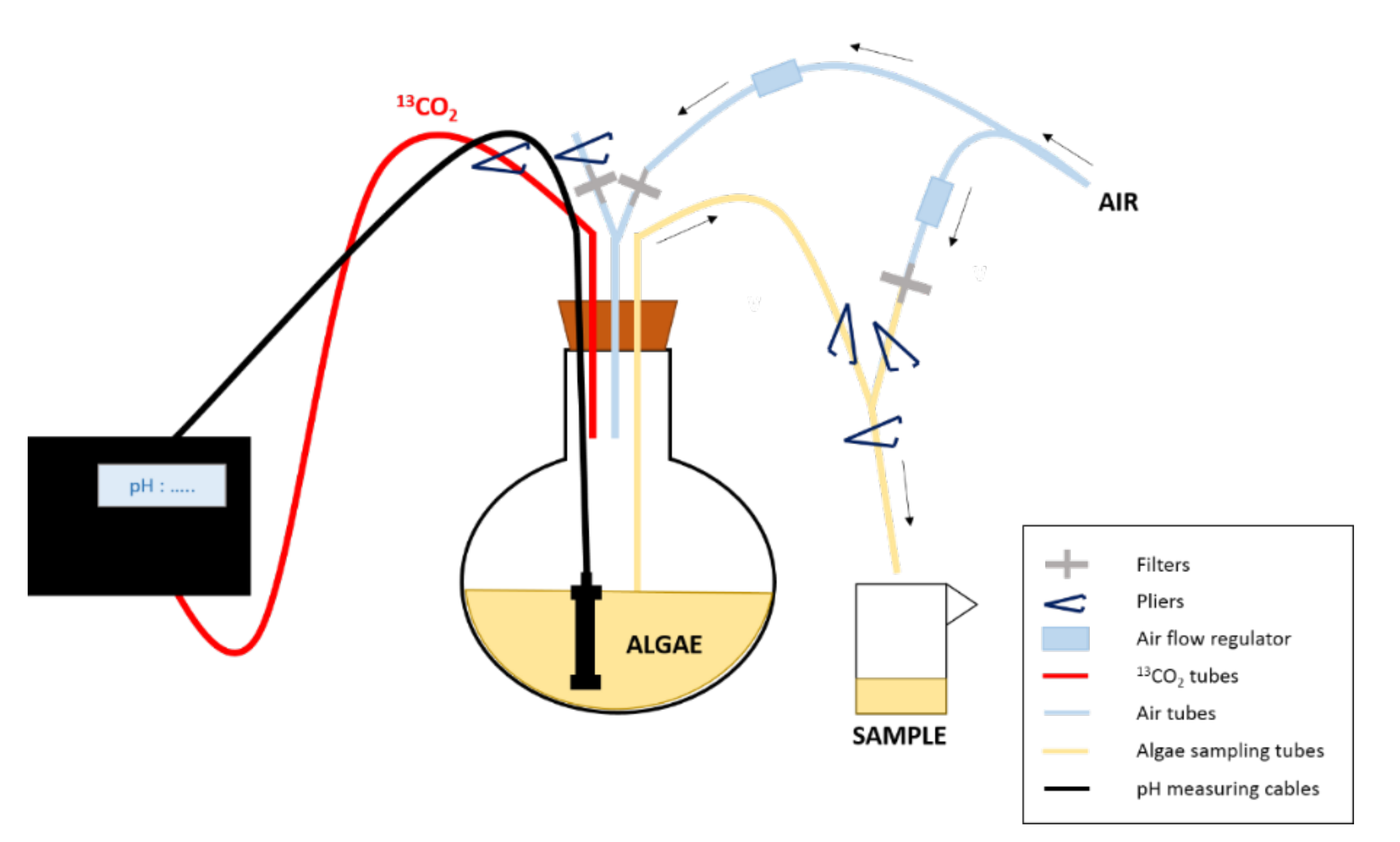
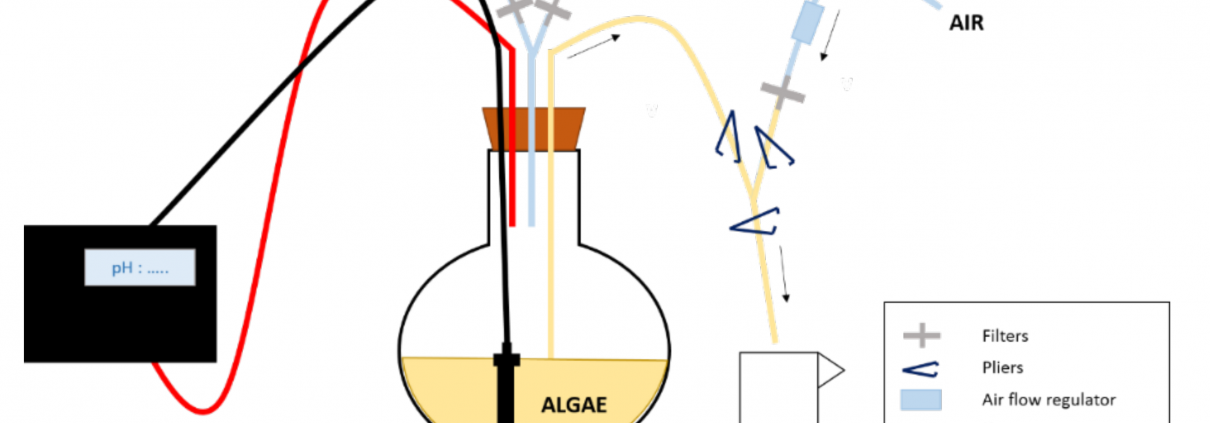
Figure 1: Experimental design of the enrichment experiment. The 13CO2 is supplied to the culture depending on its pH. To sample the algae, pliers are used to close/open the tubes/ways needed to first put the balloon under pressure and then allow sampling and finally rinse the tubes after sampling.
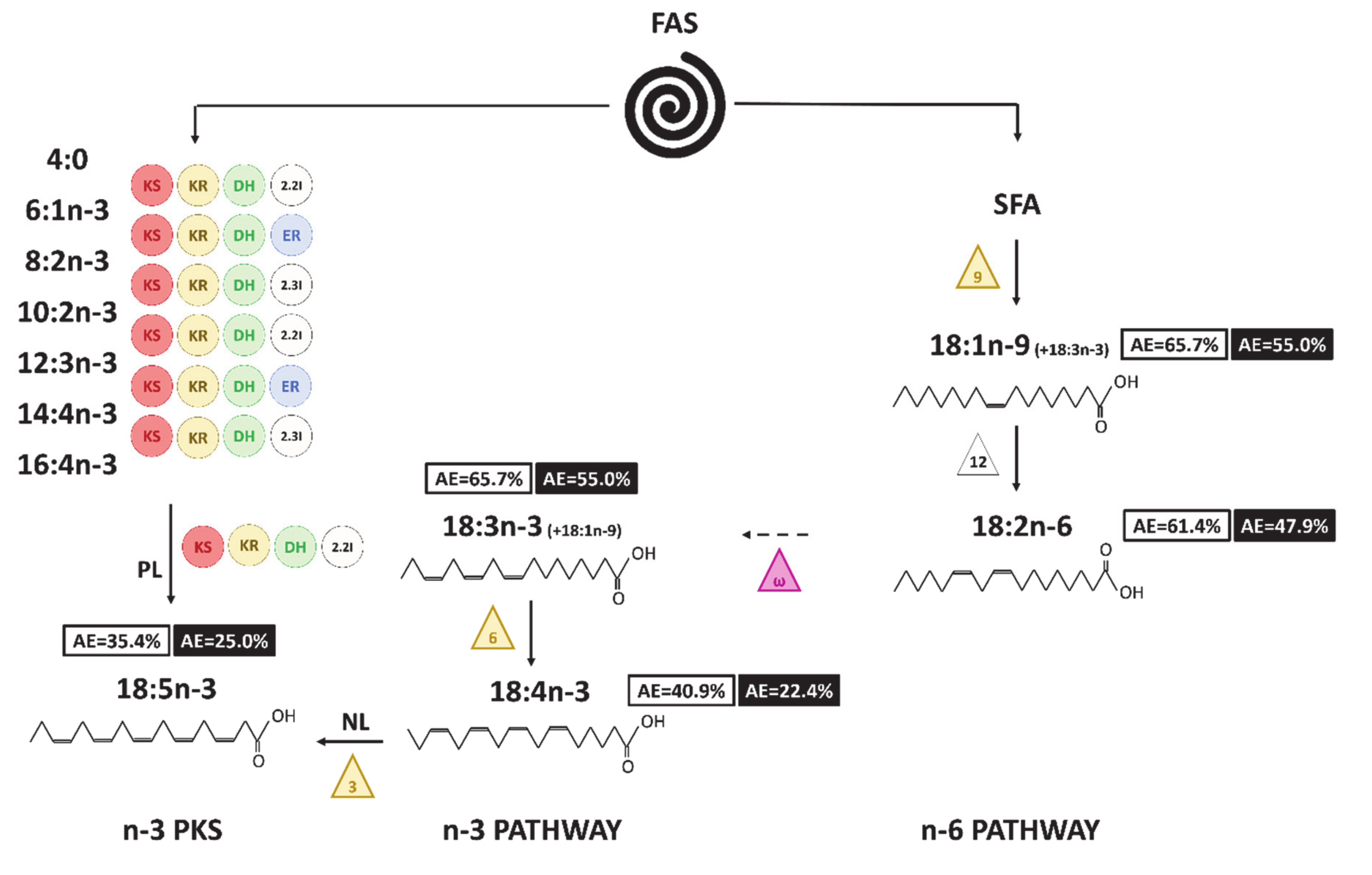
Figure 8: Hypothesized pathways to produce C18 FA in A. minutum. Numbers in boxes correspond to the final AE value (white for neutral lipids and black for polar lipids). The triangles symbolize the desaturases (front-end in yellow and methyl-end in purple), the circles the enzymes involved in the PKS pathway (KS: 3-ketoacyl synthase; KR: 3-ketoacyl-ACP-reductase; DH: dehydrase; 2.2I: 2-trans, 2-cis isomerase; 2.3I: 2-trans, 3-cis isomerase; ER: enoyl reductase). The ways with dashed arrows appear to be unlikely or cannot be proven with the enrichment dynamics. NL and PL written on the routes allowing the synthesis of 18:5n-3 indicate the fraction considered for each pathway.
Conclusions:
The fatty acid synthesis in the Dinophyte Alexandrium minutum went through different routes. The PKS pathway appeared to be a particularly fast synthetic process, responsible for the high enrichment and production of the polyunsaturated fatty acid 22:6n-3. 22:6n-3 seemed to have a central role in maintaining a good physiological state, including during encystment. In our study, we assumed that the lag phase observed reflected the possibility of temporary encystment in A. minutum. It was characterized by a significant decrease in the proportion of neutral lipids, corresponding to the consumption of the reserve lipids. The 22:6n-3 might be degraded during this process, and its degradation products might be involved in the re-synthesis of triacylglycerol during algae excystment. The enrichment dynamics of C18 PUFAs revealed that they are unlikely to be involved in the further desaturation and elongation steps of n-3 C20-C22 PUFAs. They appeared to be the final products of the classical n-3 pathway. The 18:5n-3 atomic enrichment makes possible its origin from the desaturation of 18:4n-3, the degradation of longer fatty acids such as 20:5n-3, or the PKS pathway. These C18 PUFAs have been assumed to play some role in A. minutum, such as in its toxicity. Further studies are needed to better constrain the PUFA synthesis pathway in A. minutum, and especially to further demonstrate the involvement of the PKS pathway following 22:6n-3 and 20:5n-3 synthesis.
References: